Hypervision Surgical announced that its first product has been accepted into the U.S. Food and Drug Administration’s (FDA’s) Safer Technologies Program.
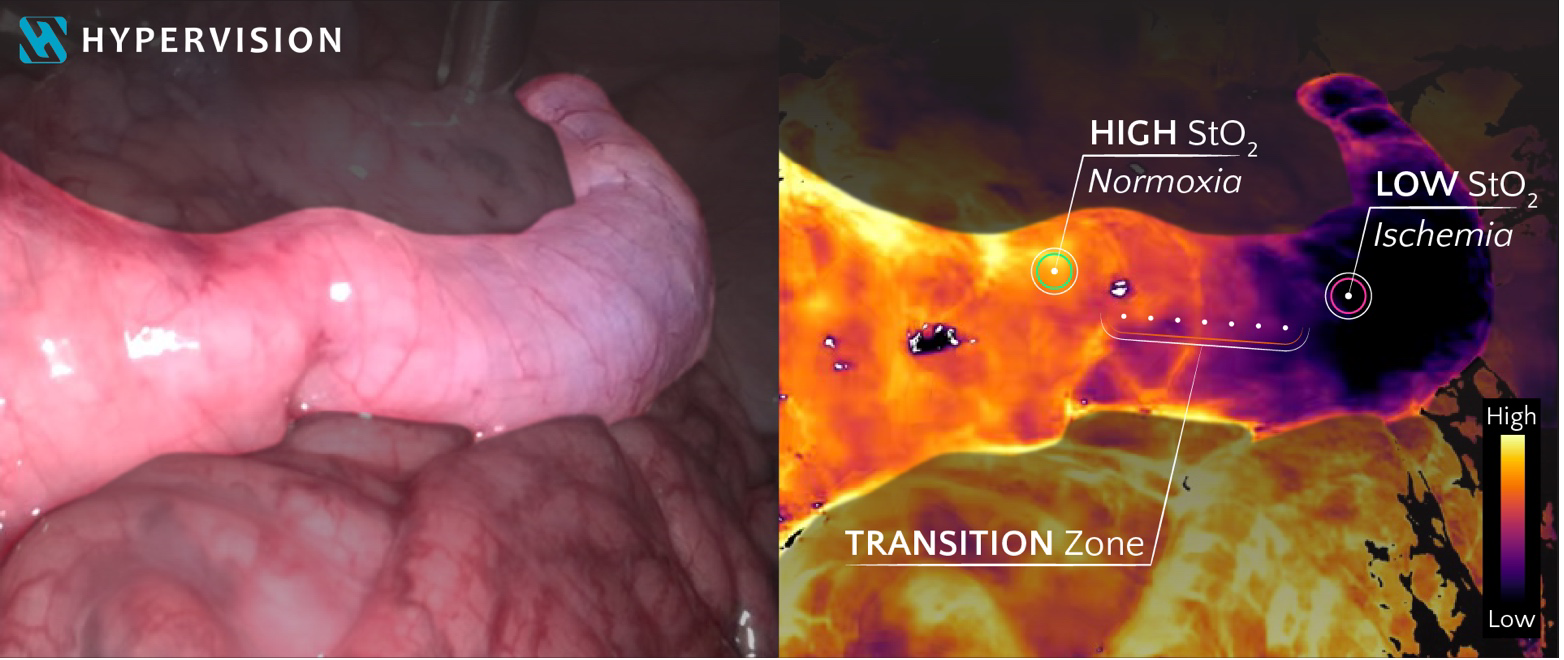
Hypervision Surgical’s technology harnesses the power of AI and hyperspectral imaging to provide live real-time RGB colour images (left) and quantitative maps of superficial tissue oxygenation (right). This extends the surgeon’s vision beyond human capabilities, enabling more informed and safer decision-making throughout surgical procedures. Images were captured in a laparoscopic pre-clinical setting with induced ischemia of the large bowel.
The FDA’s Safer Technologies Program (STeP) is designed for devices expected to significantly improve the safety of currently available treatment options consistent with the Agency’s mission to protect and promote public health. The goal of STeP is to expedite the development, assessment, and review of medical devices, ensuring expedited access for patients and healthcare providers without compromising the rigorous safety and efficacy standards required for FDA approval.
The FDA describes the STeP as “a collaborative program intended to help reduce the time it takes to develop and obtain marketing authorization for eligible devices. It offers manufacturers an opportunity to interact with the FDA’s experts through several different program options to efficiently address topics as they arise during the premarket review phase, which can help manufacturers receive feedback from the FDA in a timely way.”
Surgeons often rely on subjective judgment during surgery, resulting in poor outcomes and complications across different specialties. For instance, bowel cancer surgery carries a 10% risk of severe complications with a 35% mortality rate [1], brain cancer surgery may leave significant tumour remnants in 30% of cases [2], and prostate cancer surgery carries an 85% likelihood of post-surgery erectile dysfunction [3]. Hypervision Surgical pioneers patent-pending hyperspectral imaging technology for surgery, aiming to make surgery more precise, safer and faster. Seamlessly integrating with existing systems, its technology can enhance surgical vision across diverse specialties, from open to minimally invasive, robotic, and microscopic surgeries. By harnessing AI and clinical edge computing, Hypervision strives to revolutionize surgical outcomes and mitigate the global burden of complications for improved patient care.
We are delighted by the FDA’s recognition of the potential of our technology in significantly enhancing patient safety during surgical procedures. Being selected for the Safer Technology Program means we will receive expedited feedback from the FDA, which will further hasten the time to market for our innovation, ultimately expediting the delivery of its benefits to patients.
— Jaco Jacobs, Chief Operations Officer of Hypervision Surgical
References:
- [1] Turrentine et al, J Am Coll Surg, 2015
- [2] Fischer et al, BMC Cancer, 2017
- [3] Emanu et al, Current Opinion in Supportive & Palliative Care, 2016

