Hypervision Surgical (“Hypervision”) is thrilled to announce that its first product, the HYPERSNAP® Surgical System, has received UK Conformity Assessed (UKCA) marking. This certification confirms compliance with the highest standards of safety, performance, and innovation for medical devices in the UK market. Additionally, it marks a historic milestone as the first-ever regulatory clearance of a real-time hyperspectral imaging-based medical device designed to enhance surgical vision.
The HYPERSNAP® Surgical System has been developed to transform intraoperative visualisation by providing surgeons with real-time white-light imaging and dye-free tissue oxygenation maps. Seamlessly integrating into existing workflows, Hypervision’s hyperspectral imaging technology can enhance surgical vision across various specialties, including open, minimally invasive, robotic, and microscopic surgeries.
By harnessing AI and clinical edge computing, Hypervision aims to revolutionise surgical outcomes, reduce complications, and improve patient care globally.
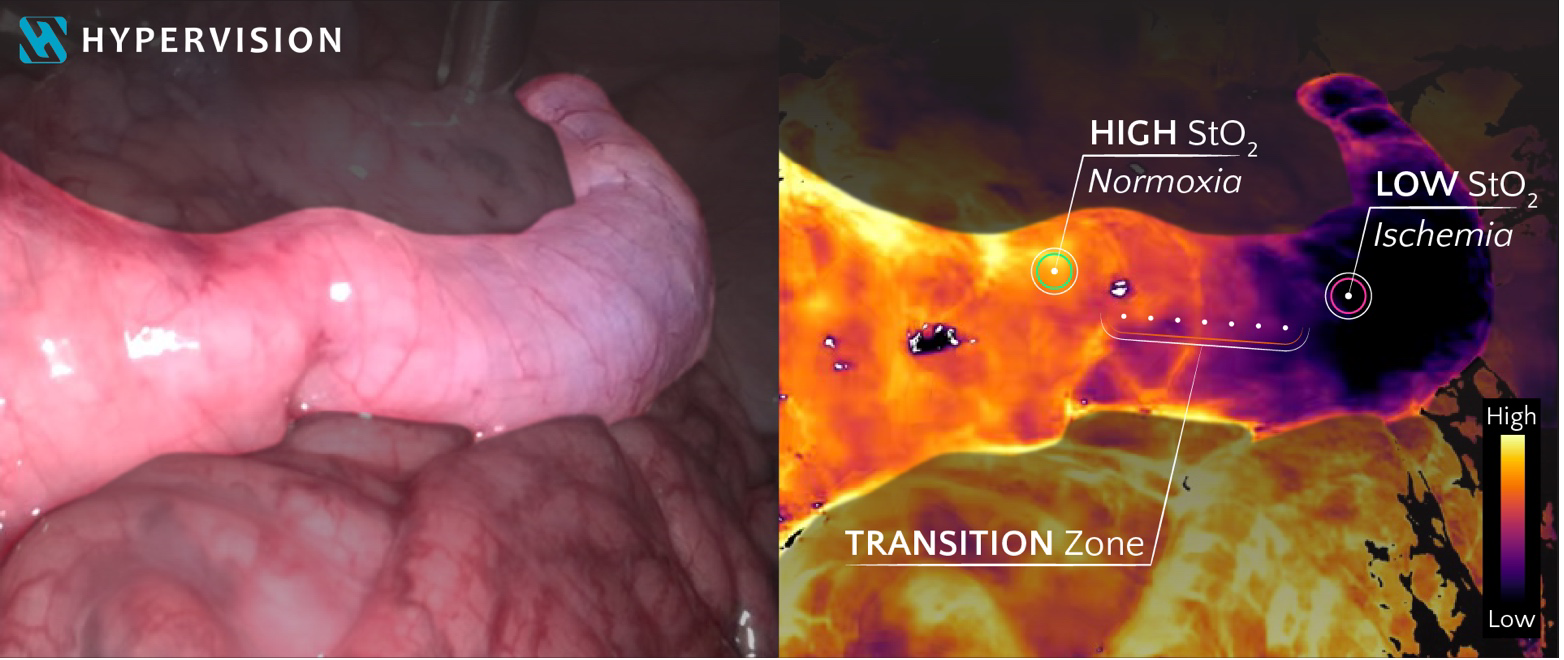
Hypervision’s technology harnesses the power of AI and hyperspectral imaging to provide live real-time white-light colour images (left) and superficial tissue oxygenation information (right). This extends the surgeon’s vision beyond human capabilities, with the potential to provide more informed and safer decision-making throughout surgical procedures. Images were captured in a laparoscopic pre-clinical setting with induced ischaemia of the large bowel.
The UKCA marking follows the inclusion of the HYPERSNAP® Surgical System in the FDA’s Safer Technologies Program (STeP) earlier this year, highlighting its global potential to improve patient safety and surgical outcomes. With this latest regulatory milestone, we are excited to accelerate our mission of delivering advanced imaging solutions to healthcare professionals worldwide.
— Jaco Jacobs, Chief Operations Officer of Hypervision Surgical

